Describe and Explain the Conditions Used in the Haber Process
2106 demonstrate understanding of the importance of a compromise between equilibrium and reaction rate in the chemical industry. The Haber process The Haber process for making ammonia provides a useful example of how this works.
Haber Process Chemistry Quiz Quizizz
The process must use high pressure because nitrogen molecules are held together with strong triple bonds.

. According to Le Chateliers Principle if you increase the pressure the system will respond by favouring the reaction which produces fewer. Produces mixture of calcium phosphate and calcium sulfate. The Haber Process task.
86 Acid a substance that neutralizes a base. The forward direction is exothermic -ve enthalpy change value. Nitrogen is supplied from the air.
Start studying Haber Process NPK Fertilisers - Major Focus. The HBP also known as the Haber process is one of the most popular ways to produce NH 3 as outlined in the previous part. 2105 describe and explain the conditions used in industrial processes for example the Haber process for the formation of ammonia and the Contact process for sulfuric acid.
The forwards direction of the Haber Process is exothermic thus in accordance with La Chateliers Principle a lower temperature will lead to an increased yield of ammonia. 200 atmospheres pressure 450C iron catalyst. The Haber process is an important industrial process which needs to be understood for A-level.
A metal is used as a catalyst in this process while maintaining high temperature and pressure. You must also be able to USE the ideas on other unfamiliar equilibria. The Haber process is an important industrial process which needs to be understood for A-level.
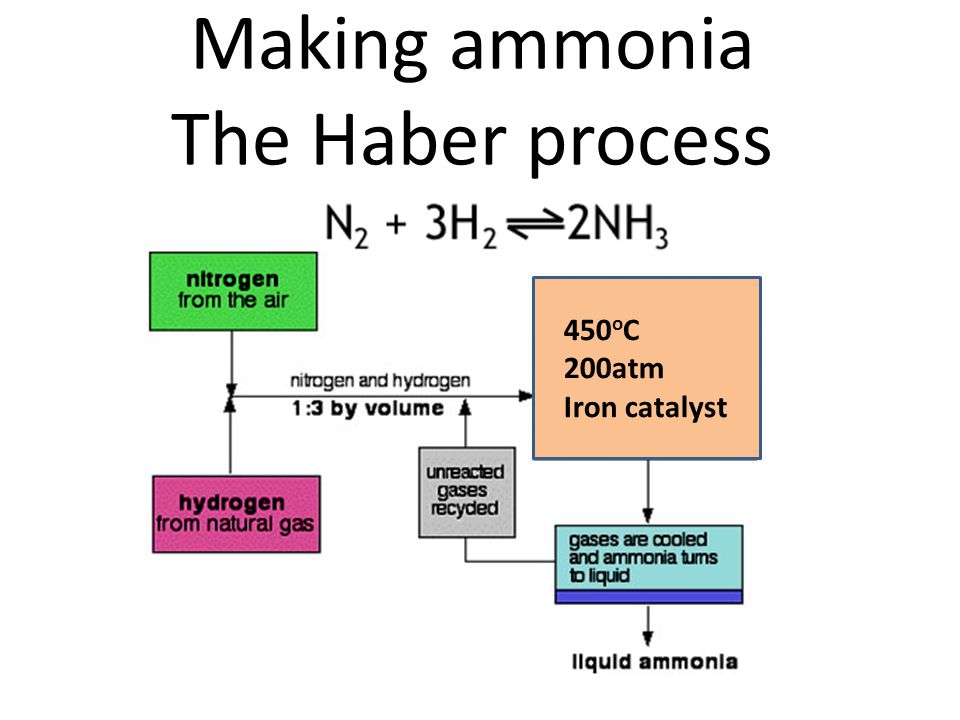
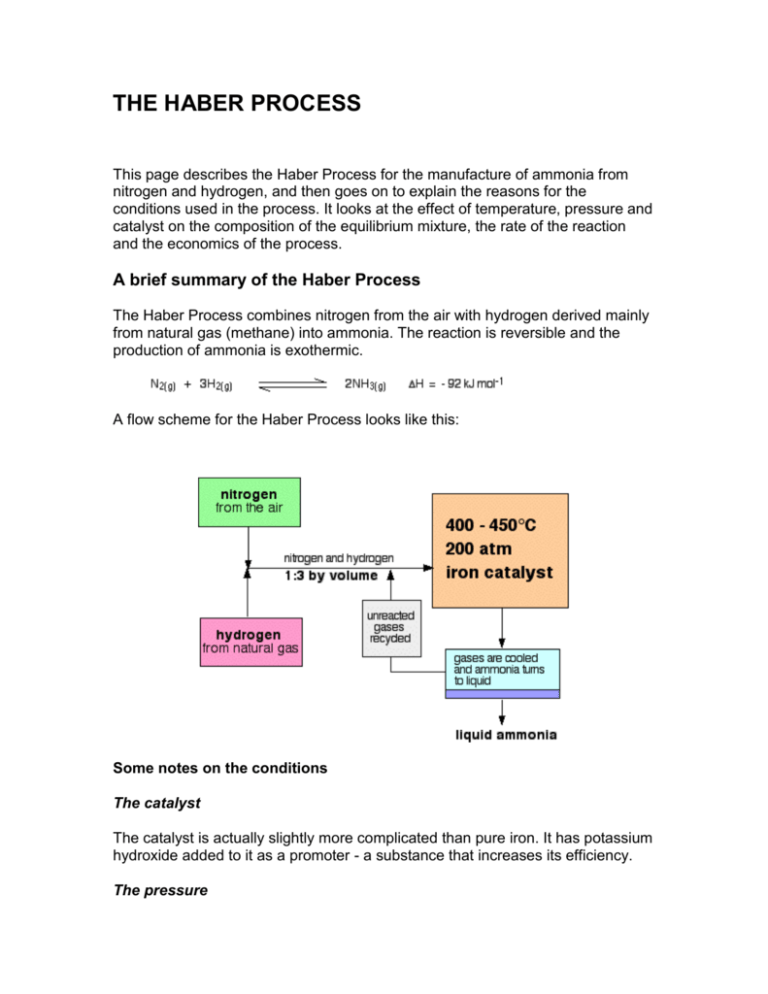
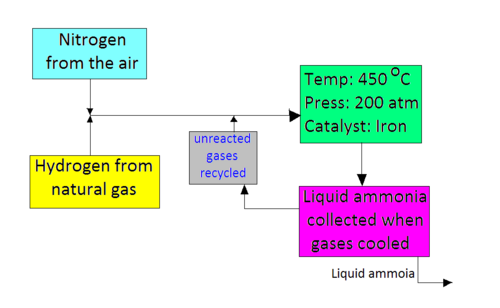
Use the equation and your knowledge of reversible reactions to explain why these conditions are used in the Haber process. The process combines nitrogen from the air with hydrogen derived mainly from natural gas methane into ammonia. The reaction is reversible and the production of ammonia is exothermic.
Up to 24 cash back Applying Le Châteliers principle to determine optimum conditions - The pressure In the reaction N2g 3H2g 2NH3g notice that there are 4 molecules on the left-hand side of the equation but only 2 on the right. However a low temperature will lead to a very slow rate of reaction thus a compromise is used of 400 degrees. He received the Nobel Prize for Chemistry in 1918 for this method which made the manufacture of ammonia economically feasible.
It looks at the effect of temperature pressure and catalyst on the composition of the equilibrium mixture the rate of the reaction and the economics of the process. EFFECT ON THE POSITION OF EQUILIBRIUM Temperature. N2g 3H2g 2NH2g forward reaction.
Bronsted-lowry acid is a proton donor. Up to 24 cash back Haber process if at too high of a temperature will have a low yield but if at low temperature will have high yield. In this Haber process ammonia is formed by the use of atmospheric nitrogen on reaction with hydrogen.
Base a substance that neutralizes an acid Acid base à salt water Alkali a base that is soluble in water. This is an equilibrium reaction N2g 3H2g2NH3g AH -92 KJ mor. These details and conditions need to be remembered.
Out of 30 marks. The forward reaction of the Haber process is exothermic heat energy released therefore the forward reaction will favour a low temperature. A State the pressure and temperature that are used in the Haber process 121 b Describe and explain why these conditions are a compromise between rate and equilibrium 19 2.
This page describes the Haber Process for the manufacture of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process. Ammonia NH is made industrially by the Haber process. Haber-Bosch process also called Haber ammonia process or synthetic ammonia process method of directly synthesizing ammonia from hydrogen and nitrogen developed by the German physical chemist Fritz Haber.
Why are the conditions used for the Haber process a compromise. The process needs a temperature of 400C and a pressure of about 150 bar to perform the reaction properly. So in the context of the Haber process the conditions which can be altered are temperature and pressure.
The Haber-Bosch process uses a catalyst or container made of iron or ruthenium with an inside temperature of over 800 F 426 C and a pressure of around 200 atmospheres to force nitrogen and hydrogen together Rae-Dupree 2011. EFFECT ON THE POSITION OF EQUILIBRIUM. The reaction generates heat in the process which can be utilized if the process is properly integrated.
Ad Learn How to Achieve Top Marks in Your Chemistry Exams From Tutors That Know. You must also be able to USE the ideas on other unfamiliar equilibria. Explain the process of treating phosphate rock with sulfuric acid 3 1.
Improve your grades with step-by-step help test prep and your own study planner. To get full marks you must consider both yield and rate of reaction in your answer. These details and conditions need to be remembered.
The conditions used in the Haber process are. The raw materials involved in the Haber process are. The forward direction is exothermic -ve enthalpy change value.
The Haber process also called the HaberBosch process is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. Due to the Haber process being a reversible reaction the yield of ammonia can be changed by changing the pressure or temperature of the reaction. Learn vocabulary terms and more with flashcards games and other study tools.
6 marks 200 atmospheres pressure. The Haber Process is used in the manufacturing of ammonia from nitrogen and hydrogen and then goes on to explain the reasons for the conditions used in the process. It is named after its inventors the German chemists Fritz Haber and Carl Bosch who developed it in the first decade of the 20th century.
Describe the conditions used in the Haber Process and explain briefly why they are used.
What Are The Conditions Required For Maximum Yield Of Ammonia By Haber S Process Quora
Comments
Post a Comment